Which of the Following Is a Buffer Solution
Mixture of salt of W_ BS_ B W_ A Buffer Transcript. A buffer is defined as the mixture of a weak acid with its conjugate base or vice versa.

Solubility Common Ion Effect Buffer Solution Buffer Solution Solubility Solutions
Maharashtra State Board HSC Science General 12th Board Exam.

. This 20 words question was answered by Heather L. 1 Point The pH response of an acid as a base is titrated in slowly added reveals Animation that the acid hom othman A. 010 M Ca OH2 and 010 M KOH 010 M LiF and O10 HF O 010 M HC2H3O2 and 010 KF 010 M HBr.
The pKb of NH3 is 474. It resists the change in p H when small amount of strong acid or base is added to it. Answered over 90d ago.
Write a balanced equation to show how the following ionic compounds dissociate in water. Choose the most correct answer. Which of the following statements correctly reflect the relationship between buffer composition and solution pH given that Ka H3OAHAH3OA-HA.
The answer is NH3 an NH4. NH3 H2O- NH4 OH- 750 mL of 0125 M HCl is added to the 100 mL of the buffer solution. Buffer solutions are obtained when a weak acid is mixed with its conjugate base or a weak base is mixed with its conjugate acid.
100 6 ratings Transcribed image text. The HCN is a weak acid but its conjugate base is CN that is not present in that solution. A buffer solution is prepared by mixing 500 mL of 0300 M NH3 with 50 mL of 0300 NH4Cl.
Buffer solutions have wide applications. NH3 is a weak base and NH4 is its conjugate acid. Who are the experts.
Which of the following processes will increase the pH of a buffer solution prepared by mixing 089 mol HCOOH formic acid and 076 mol HCOONa sodium formate into a 1-L solution. A buffer is a solution of weak acid and its conjugate base or a weak base and its conjugate acid. Hair question given is that which of following is a buffer solution so we know there are two types of buffer solution one is acidic and another is basic ok in acidic buffer this is a combination of weak acid salt of salt of weak acid strong base in basic buffer is combination of weak base salt of salt of weak base strong.
It has mass and it occupie. If the concentration of the buffer is minimize the change in pH when an higher the change in pH will be less acid or base is added to the. Which of the following is a buffer solution.
B add some HCl into the buffer solution. Hence solution of acetic acid and sodium acetate is a Buffers solution. Which of the following is a buffer solution.
It consists of a weak-acid and its conjugate base for an acid-buffer solution. 3D a add some NaCl into the buffer solution. A500 mL of 01 M CH3COOH500 mL of 01 M NaOH b500 mL of 02 M CH3COOH500 mL of 01 M NaOH c500 mL of 01 M CH3COOH 500 mL of 02 M NaOH d500 mL of 01 M CH3COOH500 mL of 01 M HCICorrect answer is option B.
C add some NaOH into the buffer solution. Buffer solutions contain a weak base and its conjugate acid or a weak acid and its conjugate base. A buffer solution is made up of acetic acid CH3COOH and sodium acetate NaCH3COO.
A buffer acts to resist GROSS changes in pH. MCQ Online Tests 73. So looking at your list a.
Has strongish acid strong base. Experts are tested by Chegg as specialists in their subject area. A buffer solution is a solution which only changes slightly when adding an acid or base to it.
Hints O HCl and NaOH O NH3 and NH4CI O HCl and KCI O NH3 and H20. IS NOT A BUFFER. Buffer solution consists of a mixture of a weak acid and its salt with a strong base.
Weak acid and a half equiv strong base. Which of the following equilibria could be used to support the claim that the addition of a small amount of NaOH to the buffer will result in only a very small change in pH. A solution that is 010 M HCN and 010 M NaCl.
A buffer solution pH buffer or hydrogen ion buffer more precisely is an aqueous solution composed of a combination or a mixture of a neutral or a very weak acid and its conjugate base or vice versa. A buffer solution is formed when appreciable quantities of a weak acid and its conjugate base are mixed together in aqueous solution. A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or weak base and its conjugate acid.
We review their content and use your feedback to keep the quality high. Buffer solutins are often able to B. The major equilibria in the buffer system are represented above.
Which of the following is a buffer solution. Which of the following is true for buffer solutions. Introduction Matter the stuff of which the universe is composed has two characteristics.
Which of the following is a buffer solution. On StudySoup on 5312017. A solution that is 010 M HCN and 010 M LiCN.
Ka HCOOH 18 x 104. Weak acid and a half equiv strong base. Acidic buffer are solution of a mixture of weak acid and salt of its conjugate base of that acid with a strong base.
Which of the following is a buffer solution. The correct option is D A C. Answered over 90d ago.
Strongish acid weak base.

Which Of The Following Is A Buffer Solution Youtube

Which One Of The Following Is Not A Buffer Solution Youtube

Which Of The Following Is The Buffer Solution Neetlab
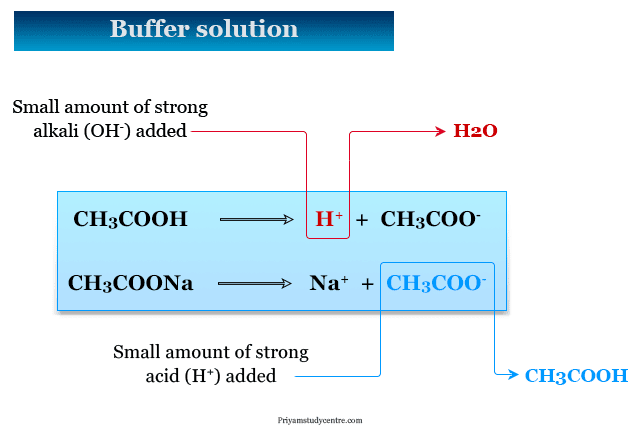
Buffer Solution Definition Types Uses

What Do You Need To Know To Calculate Ph Buffer Solution Chemistry Chemistry Review

Which Of The Following Is A Buffer Solution

Which Of The Following Pairs Will Not Form A Buffer Solution Youtube


0 Response to "Which of the Following Is a Buffer Solution"
Post a Comment